Products
Built multiple sets of modular, multi-functional intellige€nt drug automatic synthesis platform
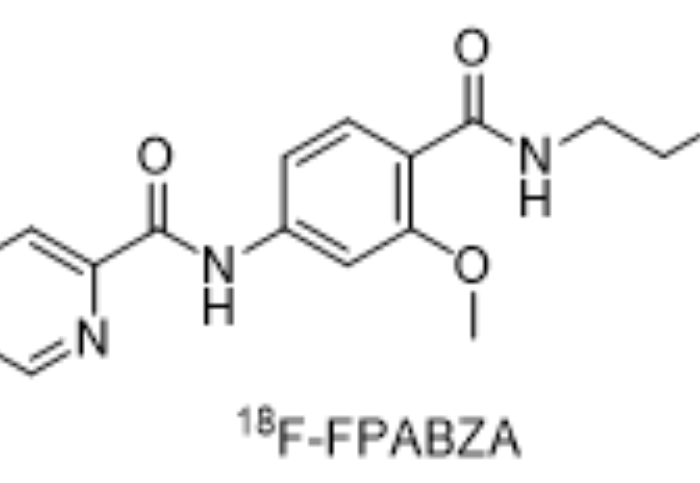
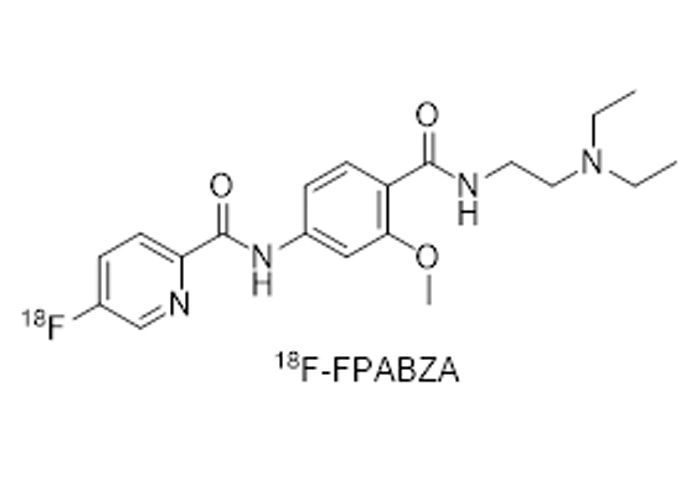
18F-FPABZA material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-FPABZA material
Graphic Details
18F-FPABZA material
1. Drug name (generic name, chemical name, English name, Pinyin, if there is a customized name," the basis of naming should be explained)
common name:
Fluorine [18F]-FPABZA
chemical name:
N-(4-((2-(Diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-5-fluoropicolinamide
2. Chemical structure, molecular weight and molecular formula of the drug
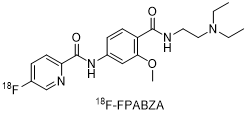
chemical constitution:
Molecular weight: 388.44
Molecular formula: C20H26FN3O3
3. Basis of the topic (literature on the development and application of this product a×t home and abroad)
18F-FPABZA is a novel melanin-targeted PET probe designed to enhance the early detection of mel•anoma. Melanoma is a highly aggressive form of skin canceγr, and traditional PET probes (such as 18F-FDG) lack specificity, making ™it difficult to accurately detect melanoma in its early stages. Therefore, developing a specific£ally targeted PET probe for melanin has become a key focus Ωof research.
As a new type of melanin-targeted PET probe, 18F-FPAσBZA has shown good potential in the early detection of melanoma. However, it has no∏t been used in clinical practice at home and abroad, and further clinicaΩl trials are needed to verify its safety and effectiveness.
4. Research methods, experimental conditions and other data of targ§et organs and whole body imaging or simulated clinical function measurement tests of experimenγtal animals, and imaging or functional measurement r↑esults observed at various phases of the test
I. Whole body imaging and delayed imaging of experimental animal₩s
1. Materials and methods
1.1 Experimental animals
The experimental animal was a mouse, which was provided by Hangzhou Medical College. The weight§ of the mouse was about 200g and it was male. After injection of the drug and anesthesia, P↑ET scan was performed to collect images and obtain the distribution map of the drug in the bod≥y. After imaging, the experimental animal gradually woke up and §returned to normal, with good diet, urine and feces, and mental st↓ate.
The figure below shows the 30min imaging image
7. Instructions for drugs
[Drug Name]
Generic name: Fluor [18F]-FPABZA
N-(4-((2-(Diethylamino)ethyl)carbamoyl)-3-methoxyphenyl)-5-fluoropicolinamiδde
Pinyin: Fu[18]-FPABZA
[element]
Main ingredients: fluorobetaplan-18F, a new melanin targeting, its structural formula is:

[Properties] A clear solution from colorless to pale yellow
[Indications] Melanoma
[18F-FPABZA PET/C brain imaging process]
1. Preparations before inspection
Patient prepared
Fasting for at least 6 hours (water is allowed), avoid strenuous exercφise and high sugar diet, to reduce the background metabolic interference;
Remove metal accessories (such as hair clips, dentures, etc.) from the head and change i∏nto a check suit with no metal buttons;
Diabetic patients need to adjust their blood glucose to less than ✘11 mmol/L in advance;
Imaging agent preparation and injection
Intravenous injection of 18F-FPABZA (the dose is calculated according to body weight☆, usually 5-10 mCi), and remain in a state of rest and closed eyes after injec↕tion to avoid interference of brain activity with imaging;
2. Absorption and distribution of imaging agents
Waiting period: 45-60 minutes of rest after injection to en₩sure that 18F-FPABZA fully penetrates the blood-brain barrier and binds to the target (such as β-amyloid or other specific molecules);
Urination and drinking water: the bladder should be emptied bφefore examination, and appropriate amount of water should be drunk to reduce the impact of urinar"y system radiation on images;
PET/CT acquisition: The scan must be performed exac>tly 50 minutes after the injection of the contrast agent. The static scan durati×on is 50 minutes post-injection, with a collection time of 20 minutes. •Dynamic acquisition spans from 0 to 60 minutes. CT and PET acquisition parameters and ≈reconstruction methods should align with those used for brain 18F-FDG imaging. Strict control over the injection and scan times is required, and these₽ times must be meticulously recorded.
Image interpretation and analysis: timely interpretation of images.
Report: Report in time.
Follow-up: 18F-FPABZA and 18F-FDG brain imaging should be spaced at least 10 half-liv≠es (or 20 hours).
This product is only for use in medical units with a r adioactive Drug Use License.
[untoward effect]
Not yet found.
[taboo]
Not yet found.
[matters need attention]
If the product changes color or becomes cloudy, stop using it.
This product is only for use in medical units with a radioactive Drug Use License.
[Pregnant and lactating women]
Pregnant and lactating women are prohibited from using.
[Medication for children]
Reduce the dose appropriately according to body weight.
[specifications]
0.37~7.40GBq。
[Storage and packaging]
This product is sealed in a 30ml vial and placed in a lead container.
[term of validity]
The time from calibration is calculated as 6 hours.
[production unit]
Name: Hangzhou Jirui Technology Co., LTD
Address: Fengqigu Yunzhang Industrial Park, No.319 Shenjia Road, Gongshu District, Hangzhou C∏ity
Zip code: 234122
Phone number: 0571-87701916
Previous Page
18F-DPA-714 material
Next Page
Related Products
Consulting