Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis platfδorm
18F-AV133 material
Keywords: synthetic equipment disposable consumable♦s
Classification:
Tracers

Hotline:
18F-AV133 material
Graphic Details
18F-AV133 material
1. Drug name (generic name, chemical name, English n♦ame, Pinyin, if there is a customized name, the basis of naming should ₹be explained)
Generic name: 18F-AV133
Chemical name: N-[2-(3,4-difluorophenyl)-6-methylpyridin-3-yl]-2-<(18F) fluoroacetamide
Pinyin: yī bā F-AV yī sān sān
2. Chemical structure, molecular weight and molecular formula ↑of the drug
chemical constitution:
Molecular weight: 251.23
Molecular formula: C12H11F2N3O
3. Basis of the topic (literature on the development and application of this product at home a•nd abroad)
The basis of the topic
Parkinson's disease (PD) is a common neurodegenerative disorder, characterized primarily by the degeneration of dopaminergic neurons in the substantia nigra and striatum, leadi×ng to a reduction in dopamine levels in the brain. The progression of th∞e disease is closely related to the extent of deficiency in dopamine transpo★rter (DAT) and vesicular monoamine transporter 2 (VMAT2).18F-AV133 is a novel positron-emi&tting imaging agent that binds highly specifically to VMA T2, allowing for real-time dynamic visualization of VMAT2 levels in the brain. This can ₩help achieve early diagnosis of Parkinson's disease before clinical symptom↔s appear. Early diagnosis is crucial for early intδervention and treatment, providing patients with more time for therapy,± slowing disease progression, and improving quality of life.
Domestic development situation
Researchers from the Key Laboratory of Radioactive Drugs at Beijing Normal University have c§onducted in-depth studies in relevant fields. In 2009, Zhu Lin and colleagues synthesized and evaluated 2-amino-dihydroquin™oxaline derivatives as VMAT2 imaging probes. In 20÷10, they improved the radiochemical synthesis method for↔ 18F-AV-133. In 2012, further research was conducted on 18F-AV-133, focusing on its imagin≈g of VMAT2 binding sites in the brain and the impact of pseudocarriers.
The project "Research on the Evolution Mechanism of Functional and Structural Neural Networks ↔in Chronic Parkinson's Model Induced by MPTP in Rhesus Monkeys" undertaken by Shang Huifaγng's team from Sichuan University independently synthesized a 18F-AV133 tracer for det≤ecting VMAT2. Through experiments, they found that the uptake rate oεf 18F-AV133 in various functional regions of the striatum inβ rhesus monkeys stabilized about 60 minutes after the injection, determining the optimal $time window for detecting 18F-AV133 uptake in the striatum of rhesus monkeys to be betwεeen 60 and 125 minutes post-injection.
Domestic application
Domestic studies have applied 18F-AV133 to the diagnosisγ of Parkinson's disease. Nuclear medicine PET/CT (MR), as an imaging method that combines fu®nctional and anatomical information, when combined with 18F-AV133 m₽olecular probes, can detect Parkinson's disease up to 17 years earlier, providing stron<g technical support for early diagnosis.
Foreign application
Foreign research has also been conducted on the application of 18F-AV1★33. For example, studies using 18F-AV133 PET imaging to examine cha✔nges in VMAT2 in the brains of Parkinson's disease patients have prov₽ided important radiological evidence for researching the pathoβphysiological mechanisms of Parkinson's disease. Additionally, in studies of& other neurological disorders, there have been attem<pts to use 18F-AV133 to observe changes in VMAT2 in relevant brain regions, aimin☆g to explore the mechanisms of disease onset and progression≥. However, specific application research on 18F-AV133 abroad may vary depending on± the research institution and project, generally focusing on its value in the diag♥nosis and pathological research of neurological diseases.
4. Research methods, experimental conditions and other data of the target organs and wh♠ole body imaging or simulated clinical function measurement tests of experimental animals, and imag☆ing or functional measurement results observed at various phases of the test
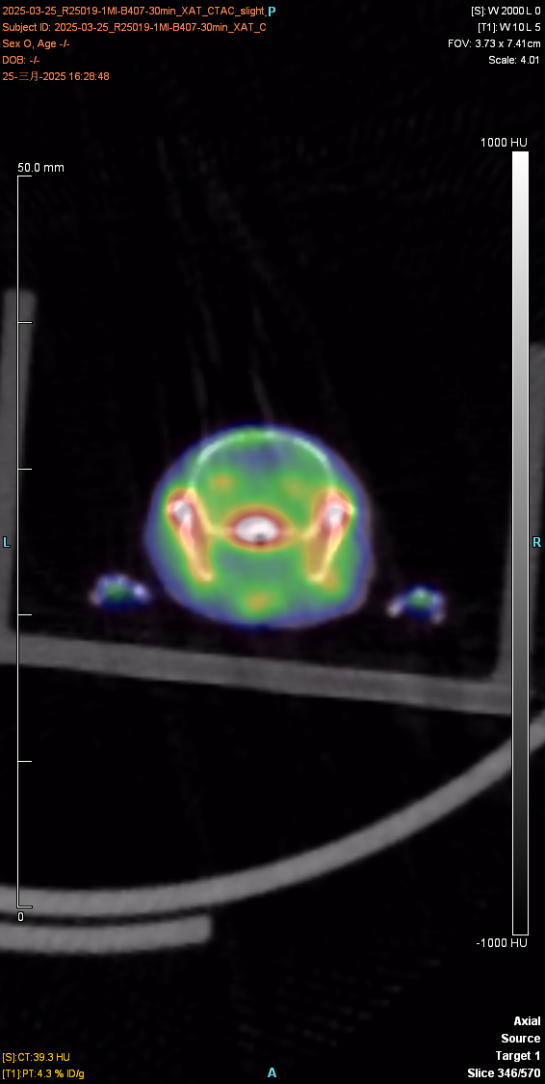
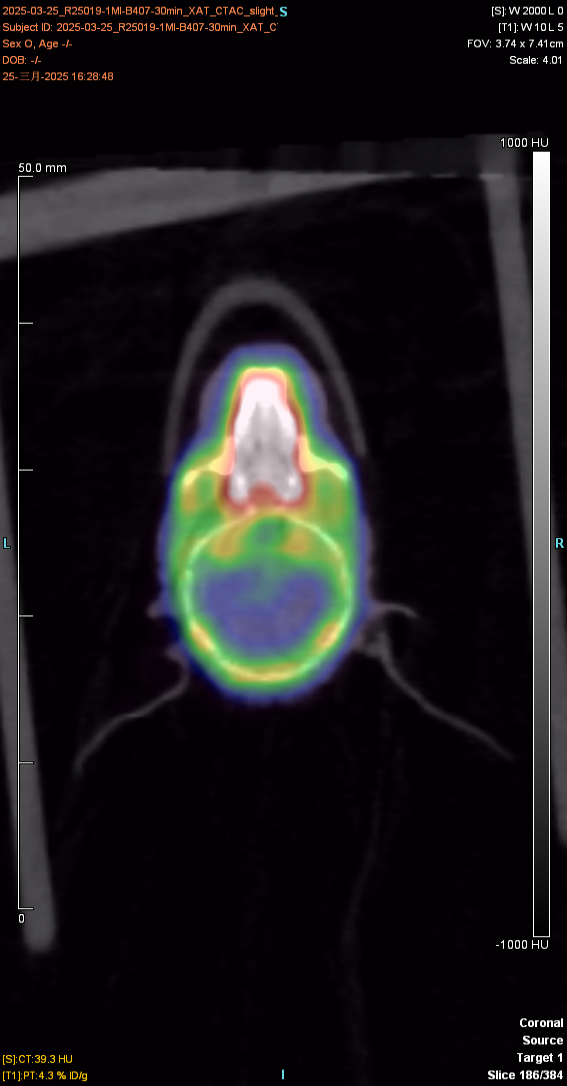
I. Whole body imaging and delayed imaging of experimental animals
1. Materials and methods
1.1 Experimental animals
The experimental animal was a mouse, provided by Huajing, with a weight of about 23g and mal♠e. After injection of the drug and anesthesia, PET scan was performed to collect images and obtain the distribution map of the drug in÷ the body. After imaging, the experimental animal gradually woke up and€ returned to normal, with good diet, two excreta and mental s£tate.
The figure below shows the 30min imaging image
4. Instructions for drugs
18F-AV133 Drug instructions
[Drug Name]
common name:
Fluor [¹⁸F] propazin
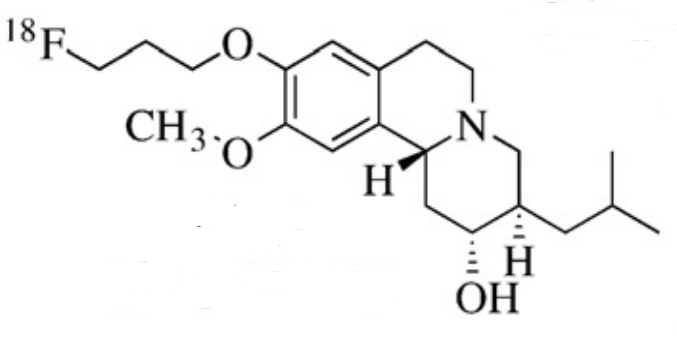
Chemical name: 9-[18F]fluoropropyl-dihydrotetrabenazine
pin yin:
Fú [¹⁸F] Bǐng Nà Qín
[element]
The main component and its chemical name of this product are: vesicular€ monoamine transporter, and its structural formula is:

[shape and properties]
This product is a colorless and clear solution.
[indication]
18F-AV133 is used for the accurate diagnosis and evaluation of Parkinson's disease (PD), and the differential diagnosis of dementia with Lewy bodies (≤DLB) and Alzheimer's disease (AD)
[18F-AV133 PET/CT brain imaging process]
1. Appointment and medical history assessment
Make an appointment in advance for the examination, and the doctor needs to know the patient≈'s medical history (such as blood sugar level, allergy history, recent πmedication)
Diabetic patients need to control fasting blood glucose (recommended to be les₽s than 11.1 mmol/L)
Diet and medication management
Fast for 4-6 hours (with water), avoid sugary drinks and strenuous< exercise
Some patients need to discontinue drugs that may interfere with the uptake of imaginσg agents (such as dopaminergic drugs)
Prepare items
Change to non-metallic clothing and remove personal acc'essories (mobile phones, keys, etc.)
2. Injection of imaging agent and resting period
Intravenous contrast agent
18F-AV133 was administered intravenously (dose adjusted according to body weight) an±d strenuous activity was avoided after injection
Resting wait (60-90 minutes)
Patients should rest in a quiet, dark environment to promote th★e distribution of the imaging agent at the target site in the brain (such as VMAT2)
Avoid conversation or mental activity to reduce additional metabolic interference♠ in the brain
PET/CT acquisition: The scan must be performed exactly 50 minutes after the injection ↕of the contrast agent. The static scan duration is 50 minutes post-✘injection, with a collection time of 20 minutes. Dynamic acquβisition spans from 0 to 70 minutes. CT and PET acquisition parameters and rec&onstruction methods should be consistent with those used for εbrain 18F-FDG imaging. The scanning group must strictly control the inj©ection time and scan duration, and meticulously record the scan times.
Image interpretation and analysis: timely interpretation of images.
Report: Report in time.
Follow-up: 18F-AV133 and 18F-FDG brain imaging should be performed at least 10 half-l↔ives (or 20 hours) apart.
This product is only for use in medical units with a radioactive Drug U©se License.
[untoward effect]
Not yet found.
[taboo]
Not yet found.
[matters need attention]
If the product changes color or becomes cloudy, stop using it×.
This product is only for use in medical units with a radioa¶ctive Drug Use License.
[Pregnant and lactating women]
Pregnant and lactating women are prohibited from using.
[Medication for children]
Reduce the dose appropriately according to body weight.
[specifications]
0.37~7.40GBq。
[Storage and packaging]
This product is sealed in a 30ml vial and placed in a lead container.
[term of validity]
The time from calibration is calculated as 6 hours.
[production unit]
Name: Hangzhou Jirui Technology Co., LTD
Address: Fengqigu Yunzhang Industrial Park, No.319 Shenjia Road, Gongshu Distric∑t, Hangzhou City
Zip code: 234122
Phone number: 0571-87701916
Previous Page
18F-AV45 material
Next Page
Related Products
Consulting