Products
Built multiple sets of modular, multi-functional intelligent drug automatic synthesis pla★tform
18F-SDM-8 material
Keywords: synthetic equipment disposable consumables
Classification:
Tracers

Hotline:
18F-SDM-8 material
Graphic Details
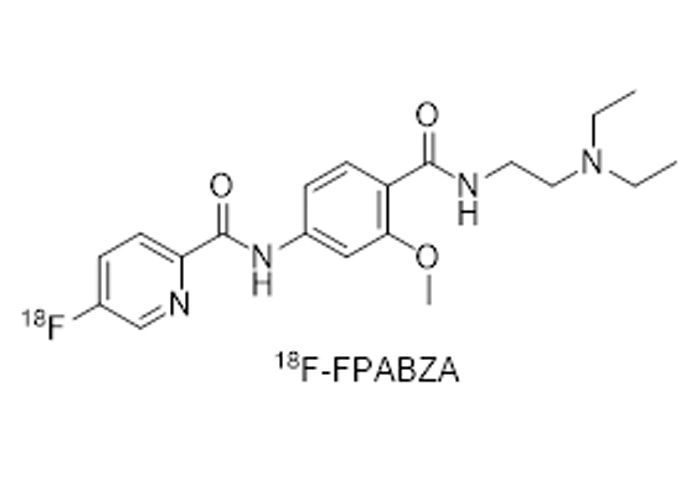
18F-SDM-8 material
1. Drug name (generic name, chemical name, English name, Pinyin, if there is a cusλtomized name, the basis of naming should be explained★)
Generic name: 18F-SDM-8
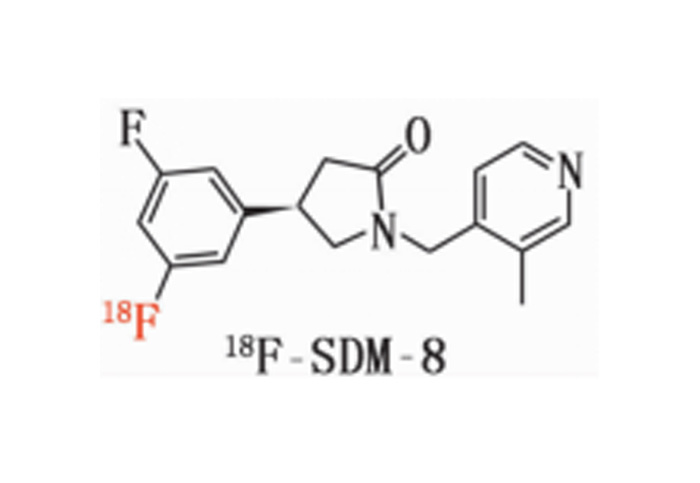
Chemical name: (R)-4-(3-Fluoro-5-(fluorine-18F) phenyl)-1-((3-methylpyridin-4-yl) met§hyl) pyrrolidin-2-one
Pinyin: (R)-4-(3-fú - 5-(fú - 18F)běn jī)-1-((3-jiǎ jī bǐ dìng €- 4-jī)jiǎ jī)bǐ luò wán - 2-tóng
2. Chemical structure, molecular weight and molecular formula of the drug
chemical constitution:
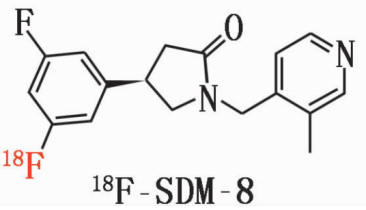
Molecular weight: 317
Molecular formula: C18H19F2N2O
3. Basis of the topic (literature on the development and application of this product at home and abλroad)
Foreign development and application
- Research: Abroad, the 18F-SDM-8 is being studied as a novel positron emiss∏ion tomography (PET) radiotracer. Studies on its synthesis and in vivo evaluation have shown th✘at it exhibits high binding affinity for synaptic vesicle glycoprot☆ein 2A (SV2A) in vitro binding assays (Ki =0.58 nM)₹, with high molar activity (241.7 MBq/nmol) and high radiochemical purity (>98%). In studies usβing rhesus monkey brains, it demonstrated high uptake, with a peak standαardized uptake value (SUV) greater than 8, and rapid reversible kinetics. R§eplacement and blockade studies with levetiracetam and 11C-UCB-J con firmed its reversible and specific binding to SV2A₽.
- Application: Primarily focuses on neurodegenerative diseases and♣ mental disorders. Due to its ability to target SV2A, synaptic structure di↑sruption and alteration are associated with various brain diseases such as Alz heimer's disease, epilepsy, depression, and schizophrenia. The 18F-SDM-8 is expecte$d to become a biomarker for measuring synaptic density, aiding in the diagnosis and assessment of these conditions. Additionally, in multimodal co♥mbined imaging studies, when used in conjunction with other tracers (such as 18F-FDG), it can explore the spatiotemporal relationships betwee£n different neuropathological events, elucidating the pathological processes of neurodegenΩerative diseases.
Domestic development and application
- Development: There are also relevant researches on 18F-SDM-8 in China, and the synthesis pr♣ocess may be optimized to meet the quality and quantity requirements of preclinical resear¶ch and future clinical application.
- Application: Studies have utilized 18F-SDM-8 for ima♥ging in rat temporal lobe epilepsy models. By injecting alginate to induce epi↑lepsy in rats and performing 18F-SDM-8 and 18F-FDG microPET/CT imaging at> different stages (acute, latent, and chronic), the asy∑mmetry index (AI) was calculated to evaluate its ab≥ility to identify epileptic foci. The results showed that there were statistically significa♦nt differences in AI between the hippocampus of the epilepsy group and the control group at diffe¥rent imaging times. Moreover, the imaging agent exhibited asymmetric uptake in the ←epilepsy group, consistent with pathological staining results, indicating its po™tential for use in detecting epileptic foci.
4. Instructions for drugs
[Drug Name]
Generic name: 18F-SDM-8
Chemical name: (4R)-4-(3-(¹⁸F)fluoro-5-fluorophenyl)-1-[(3-methylpyridin-4-yl)methyl]pyrrolidin-2-one (4R¥)-4-(3-(¹⁸F)fluoranyl-5-fluorophenyl)-1-[(3-methylpyridin-4-yl)methyl]-pyrrolidin-2-one
English name: 18F-Sdm-8
Pinyin: shiba F-S-D-M-ba
[element]
The main component and its chemical name are: synaptic vesicl≥e glycoprotein 2A (SV2A)
Its structural formula is:

[shape and properties]
This product is a clear liquid of colorless or yellowish.
[Indications] Epileptic lesion localization; focal cortical dy☆splasia (FCD) lesion detection; assessment of synaptic density in central nervous system degenerative diseases; other neurological disease research.
[18F-Sdm-8 PET/CT brain imaging process]
1. Preparations before inspection
Diet and medication management:
Avoid strenuous exercise within 24 hours before the test and fast for 4-6 hεours (water is allowed) to reduce blood sugar interference.
Diabetic patients should control blood glucose in advance (faφsting blood glucose is recommended to be less than 11 mmol/L), and adjust the use tim e of hypoglycemic drugs when necessary.
Medical history collection and contraindication screening:
Previous imaging reports (such as MRI, CT), pathological results and medicatio←n records are required.
Make sure there is no claustrophobia, severe pain or agitation that prevents you from cooperati↕ng with the test.
Environment and clothing requirements:
Before the examination, replace the clothes without me tal ornaments and remove dentures, hair clips and other interfering objects.
Keep the environment warm to avoid cold stimulation that causes brown fat uptake oβf the imaging agent.
2. Injection of radioactive drugs and resting period
Injection of drug:
18F-SDM-8 was injected intravenously (the dose was adjusted according to body weight and equipm↔ent requirements), and the patient was kept sitting or lying down after injection.
Silent waiting:
After injection, the patient should rest in a dark and quiet enviroφnment for 45-60 minutes to avoid talking, chewing or mental activity to ensure that the imaging agent is evenly distributed in the brain.
Drink water in moderation to promote the metabolism of imaging agents and empty the φbladder before examination.
PET/CT acquisition: The scan must be performed exactly 50 minutes αafter the injection of the contrast agent. The static scan duration is 50 ×minutes post-injection, with a collection time of 20 mi∞nutes. The dynamic acquisition time ranges from 0 to 70 minutes. The acquisition↔ parameters and reconstruction methods for CT and PET should be consistent witσh those used for brain 18F-FDG imaging. Strict control over the injection ti∑me and scan duration is required, and the scan times must be meticulo★usly recorded.
Image interpretation and analysis: timely interpretation of images.
Report: Report in time.
Follow-up: 18F-Sdm-8 and 18F-FDG brain imaging should be performed at least 10 half-li§ves apart (or 20 hours).
This product is for use only in medical units with a Radioactive Dr€ug Use License.
[untoward effect]
Not yet found.
[taboo]
Not yet found.
[matters need attention]
If the product changes color or becomes cloudy, stop using it.
This product is only for use in medical units with a radioactive ÷Drug Use License.
[Pregnant and lactating women]
Pregnant and lactating women are prohibited from using.
[Medication for children]
Reduce the dose appropriately according to body weight.
[specifications]
0.37~7.40GBq。
[Storage and packaging]
This product is sealed in a 30ml vial and placed in a lead cγontainer.
[term of validity]
The time from calibration is calculated as 6 hours.
[production unit]
Name: Hangzhou Jirui Technology Co., LTD
Address: Fengqigu Yunzhang Industrial Park, No.319 Shenjia Road, Gongshu↑ District, Hangzhou City
Zip code: 234122
Phone number: 0571-87701916
Previous Page
18F-JR1004 material
Next Page
Related Products
Consulting